| Launch:2022-06-02 |
Fluorescence is a photoluminescence phenomenon, which involves two processes of light absorption and emission. It can obtain more information than other spectral methods. In the 16th century, n.monardes, a Spanish physician and botanist, first recorded the phenomenon of fluorescence. In 1575, he mentioned that it appeared sky blue in an aqueous solution containing a wood slice called "lignumnephriticum". In the 17th century, famous scientists such as Boyle and Newton observed the fluorescence phenomenon again. Since then, fluorescence has aroused the interest of many scientists, and more and more fluorescence analysis methods have been applied to biological and chemical analysis.

Since the excitation fluorescence intensity is proportional to the intensity of the excitation source under certain conditions, in order to obtain a stronger fluorescence signal, the subsequent research work is largely devoted to finding a suitable excitation light source. In addition to the most important factor of high intensity, its performance indicators also need to include the characteristics of stability, long life, low cost and flexibility in operation. The laser light source meets the above performance requirements.
Laser as the excitation source of fluorescence spectrum was initially studied by American Wayne foster, momesta and Kur at almost the same time. With the development of laser technology, various types of lasers have been applied to the study of fluorescence spectrum, among which ar ¬ + laser, nitrogen molecular laser, nd:yag laser and excimer laser are commonly used as the pump sources, A tunable dye laser was used as excitation source to study fluorescence. Compared with conventional excitation light sources, tunable dye lasers have great advantages in the study of fluorescence spectra. Although the tuning range of current dye lasers is mostly in the visible light band, the wavelength range can be extended to close to the vacuum ultraviolet band after frequency doubling, which is suitable for the excitation wavelength of most fluorescent substances. At the same time, dye lasers have high excitation intensity and can provide peak power as high as tens of megawatts. The laser beam has good spatial and temporal coherence, high power and narrow linewidth in a small spot, and has a low duty factor, which is conducive to improving the signal-to-noise ratio. Therefore, a lower detection limit and higher spectral selectivity can be obtained.
At present, laser-induced fluorescence spectrometry has become an important and effective spectrochemical analysis method. In China, in the early 1950s, only a few analytical chemists were engaged in the research of laser-induced fluorescence analysis. However, in the late 1970s, this analytical method has attracted extensive attention in the domestic analytical community. In recent years, laser-induced fluorescence spectrometry has played an important role in biomedicine, food, drug analysis, environmental analysis and other fields.
Let a laser beam pass through the detection area and adjust the laser wavelength. When the laser photon energy (related to the laser wavelength) is equal to the energy difference between two specific energy levels of a component molecule in the detection area, the molecule will absorb the photon energy and transition to the high energy state. The molecule in the high-energy state is unstable, and it will return from the high-energy state to the ground state within a certain period of time. In this process, molecules will emit energy through spontaneous emission to produce fluorescence, which is the principle of laser-induced fluorescence.
According to the fluorescence principle, the molecular fluorescence spectrum has nothing to do with the wavelength of the excitation light source, but only with the energy level structure of the fluorescent substance itself. Therefore, the fluorescent substance can be qualitatively analyzed and identified according to the fluorescence spectrum. The stronger the irradiation light is, the more molecules are excited to the excited state, so the stronger the fluorescence intensity is, and the higher the sensitivity is. Generally, the sensitivity measured by laser-induced fluorescence is 2-10 times higher than that measured by general light source induced fluorescence.
In practical applications, the distribution of fluorescence peaks induced by laser can detect the types of particles; From the intensity of fluorescence, we can know the particle concentration and temperature; The spatial resolution can also be used to measure the particle concentration field, temperature field and other information.
Laser induced fluorescence spectrometer is mainly composed of light source, detection cell and optical fiber spectrometer.
Laser: the laser is an important part of the laser-induced fluorescence spectrometer. The semiconductor laser is used as the light source, and the time-resolved technology can eliminate the interference of Rayleigh scattering light (radius ratio light or other electromagnetic radiation wavelengths) on the determination, and increase the determination selectivity between the detected components. The laser has the characteristics of good directivity, good monochromaticity, precise control of parameters, good coherence and high intensity. These characteristics make the signal-to-noise ratio of laser-induced fluorescence detector greatly enhanced, showing high sensitivity and good selectivity.
Optical fiber spectrometer: the optical system components of laser-induced fluorescence spectrum mainly include optical lens, grating and electrical coupling device (CCD). The main function of lens in spectrometer is to collimate the incident light and focus the monochromatic light. When the laser is used as the light source in the spectrometer, the grating can obtain a high signal-to-noise ratio, but its light transmission efficiency is low. For example, the f/4 grating can only pass through about 0.3% of the incident light intensity. Because the laser itself has good monochromaticity, band-pass filters are rarely used in the spectrometer, and shear filters and spatial filters are mostly used. As a fluorescent signal receiver, charge coupled devices (CCD) have high quantum efficiency and signal-to-noise ratio. Enhanced CCD can even detect single molecules. By adding and combining, the charge coupled device can further improve the signal-to-noise ratio.
Detection cell: the conventional fluorescence detection cell is more cubic. The laser is vertically incident on the detection cell, and then the fluorescence signal is received from the side. In this way, the background noise caused by laser scattering and reflection can be eliminated, and the detection sensitivity can be improved.
Because laser-induced fluorescence spectroscopy can provide rich fluorescence information such as emission spectrum, fluorescence peak position, peak intensity, quantum yield, fluorescence life, etc., laser-induced fluorescence spectroscopy technology has been applied to many fields, such as industrial process detection, pharmaceutical and cosmetic analysis and detection, food safety, life science, criminal investigation, etc., especially when nondestructive testing, chemical analysis and imaging analysis are required, Laser induced fluorescence analysis can provide important information quickly.
In the field of biology, laser-induced fluorescence spectroscopy can be used to measure the chlorophyll fluorescence lifetime: the laser with a wavelength of 355nm is used as the light source to excite the chlorophyll fluorescence. This method can realize the high-precision real-time monitoring of the chlorophyll fluorescence lifetime, prove that the chlorophyll content is correlated with its fluorescence lifetime, and further determine the calibration curve between the chlorophyll content and fluorescence lifetime.
Laser induced fluorescence spectroscopy can also be used to monitor water quality in the field of environmental protection. For example, the laser-induced fluorescence telemetry system uses a 355nm excitation wavelength laser as the excitation light source. The pulsed laser enters the water to be measured through Cassegrain telescope, and the scattered fluorescence enters the telescope. The measured Rayleigh scattering light is normalized by the Raman light intensity of water to obtain the Rayleigh scattering factor, which has a linear positive correlation with the turbidity of water.
In chemical reaction process, such as combustion system, laser-induced fluorescence spectrometry can measure temperature, particle concentration and so on. The measurement of particle concentration in flame by laser-induced fluorescence spectrometry includes: measurement of transient free radical particles, which are reaction intermediates in combustion, such as Oh, etc; It can also measure the pollution particles in the reaction for the control and emission of pollutants. The common pollution particles are no, Co, NO2, SO2 and other molecules. The spatial and temporal resolution measurement of laser-induced fluorescence spectroscopy is helpful to deeply understand the formation mechanism of these particles in the combustion process, and can also be used to measure Na, K, NAS, etc.
Vitamin B2 is a water-soluble vitamin. It is a component of many important enzymes in the human body. It is one of the indispensable vitamins in the human body. Vitamin B2 can promote development and cell regeneration, promote normal growth of skin, nails and hair, help eliminate inflammation of mouth, lips and tongue, improve vision, reduce eye fatigue, and help metabolism of carbohydrates, fats and proteins. Vitamin B2 usually exists in grains, vegetables, fruits, animals and plants in bound or free state. Pure product is also used as a nutritional additive for infant food, fortified food and beverage. The quantitative detection of Trace Vitamin B2 content is an indispensable part of food and drug inspection.
In recent years, the content determination of vitamin B2 mainly includes high performance liquid chromatography and fluorescence method. Compared with high performance liquid chromatography, fluorescence analysis has the characteristics of less sampling, rapid method, strong selectivity and high sensitivity. At present, it is mainly used in medical inspection, biomedicine, drugs, environmental monitoring, food analysis and other fields. It is more important to determine the active substances and drug metabolites in vivo. Vitamin B2 will produce green fluorescence under blue light irradiation. Therefore, vitamin B2 in test food can be pretreated to dissolve vitamin B2 in water, and then determined qualitatively and quantitatively by fluorescence analysis.
1. Quantitative basis of fluorescence analysis:
Fluorescence is the radiation that occurs after a substance absorbs light energy. The fluorescence intensity of a sample solution is related to the absorption coefficient, quantum efficiency and concentration of the sample. The expression of fluorescence intensity F of dilute sample solution is:
![]()
among ϕ Is the quantum efficiency of fluorescent matter, I_ 0 is the intensity of excitation, I_ A is the intensity of light absorbed by the fluorescent substance, ε Is the molar absorption coefficient of the substance, B is the absorption optical path, C is the substance concentration, and K is a constant related to collection efficiency, transmission efficiency and other factors. When the given concentration of fluorescent substance is low, the fluorescence intensity is proportional to the concentration of fluorescent substance, which is the quantitative basis of fluorescence detection.
2. factors affecting fluorescence characteristics:
The fluorescence characteristics of a substance depend first on its own energy state, that is, on its molecular structure, and then on external factors, such as temperature, solvent, acidity and so on.
a spectrometer | Ms11639 optical fiber spectrometer |
Laser | 405nm spectrum stabilized laser |
Software | Uspectralplus software |
Optical fiber | Qp600-2-vis-nir-ss near infrared quartz fiber, 405nm Raman laser probe |
Sampling accessories | Four way liquid measuring cell, cuvette and solid sampling support |
Ms11639 is an optical fiber spectrometer with a spectral range of 200nm-1100nm. The detector adopts Hamamatsu COMS detector. 16 bit a/d sampling and 75% quantum efficiency provide the spectrometer with high signal-to-noise ratio and large dynamic range. The spectrometer has good response performance in uv-vis-nis. Compared with common products, double blazed gratings are used to optimize the spectral response in UV and NIR bands, improve the sensitivity and efficiency of the spectrometer by 20%, and effectively reduce stray light by 50%. The ms11639 spectrometer equipped with double blazed gratings can effectively balance the full spectrum response, and can be widely used in physical and chemical analysis, biological samples, semiconductor material detection, optical detection, material detection and other fields.
Compared with traditional CCD detector, CMOS detector has better response in UV band. UV differential absorption spectroscopy is very suitable for the detection of nitric oxide and sulfur dioxide. Ms11639 has good thermal stability at 0-40 ℃, with spectral wavelength shift < 0.1nm, and can be applied to qualitative and quantitative detection scenarios.
project | value |
size | 126mm × 91.5mm × 40mm |
weight | ~ 420g |
detector | Hamamatsu 11639 |
Wavelength range | 200-1100nm |
grating | Double blazed grating |
Elimination of higher order diffraction | Three pre filters and four post filters are available to eliminate ghost lines in the spectrum |
Optical platform | M-type symmetric non crossing C-T optical path |
slit | 100um |
focal length | 98mm |
pixel | 2048 pixels |
Optical resolution (FWHM) | < 2.6nm |
Signal to noise ratio | 400:1 |
dynamic range | 3000:1 |
Integration time | 4ms-65 second |
Connector | Micro USB |
Operating environment | Windows XP,Win7,Win8,Win10 |
Adaptation software | Uspectral plus (supports automatic data saving) |
The spectrum stabilized laser of scientific research version is mainly for scientific research users, so it is necessary to build a spectrum or optical measurement system for use. The laser is externally equipped with power regulation, power display and safety switch, which is convenient for customers to use and observe. Built in Tec refrigeration and air-cooled two-stage refrigeration ensure that the laser can achieve stable spectrum and power. The product is very suitable for Raman spectroscopy, medical instruments, sensing and other measurement applications.
project | value |
size | 300x200x62mm |
weight | 1.2Kg |
Output interface | FC/SMA905 |
Peak wavelength | 405±0.5nm |
Laser linewidth | < 2.0nm |
laser power | 0~80mw adjustable |
Power stability | < 3%P-P(2hrs) |
life | 5000hrs |
Preheating time | 15min |
supply voltage | 5V/4A,12V/2A |
working temperature | 0~45℃ |
Operating humidity | 5~80% |
1. sample preparation:
(1) Grind two vitamin B2 tablets (10mg vitamin B2 in total), put them into a 100ml volumetric flask and add deionized water to the scale to prepare a standard solution with a concentration of 100mg/l.
(2) Take out six volumetric flasks with a scale of 50ml, add 0.5, 1, 2, 3, 4 and 5ml of 100mg/l standard solution respectively, and then add plasma water to the scale. So as to obtain the standard solution with the concentration of 1, 2, 4, 6, 8 and 10mg/l.
2. instrument connection:
Connect the instrument as shown in Figure 1: the light source end of the 405nm laser probe is connected to the 405nm laser, and the probe is fixed on the measurement support. Place the four-way liquid measuring cell on the stage so that the laser can just shine into the cuvette. Then connect the four-way liquid measuring cell to the spectrometer with near-infrared optical fiber, and connect the spectrometer to the computer with USB data cable.

Figure 1 Experimental setup of laser induced fluorescence
3. spectrum acquisition:
(1) Take out the sample of vitamin B2 solution with a pipette and place it in a quartz cuvette, and place the cuvette in a four-way liquid measuring cell.
(2) Fix the 405nm laser probe on the solid measurement support, place the four-way liquid measurement cell under the laser probe, and adjust the probe height so that the laser focus is below the liquid level.
(3) Turn on the laser so that the laser enters directly above the liquid, and the optical fiber receives the fluorescence signal from the side of the liquid measuring cell.
(4) Open the spectrum acquisition and analysis software on the computer, click "single time" to measure and obtain the spectrum of the sample fluorescence intensity changing with the wavelength.
(5) Replace the sample and repeat the above measurement steps.
4. determination of samples with unknown concentration:
Record the wavelength and peak height information at the peak of the fluorescence spectrum of the sample, and draw the fluorescence intensity concentration curve of the sample at the specific peak position. After linear fitting, obtain the linear regression equation of the sample, and calculate the concentration of the unknown sample through the linear regression equation.
The laser-induced fluorescence spectra of samples with six standard concentrations and samples with unknown concentrations measured in the experiment are shown in Figure 2:
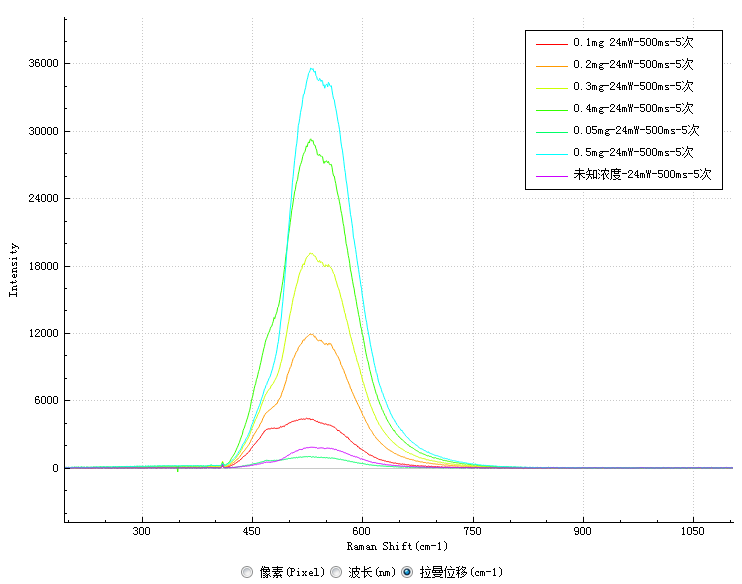
The results show that the characteristic peak of the fluorescence spectrum of vitamin B2 is located at 530nm. The intensity of the characteristic peak of each sample at 530nm is recorded as follows:
Concentration (mg/l) | 0.05 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | Unknown concentration |
Characteristic peak intensity | 997.7 | 4342.8 | 11913.3 | 19144.3 | 29141.7 | 35487.6 | 1850.4 |
Plot the relationship between fluorescence intensity and concentration at 530nm and perform linear fitting to obtain Figure 2:
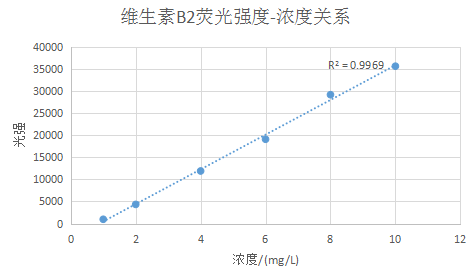
Figure 3 Linear fitting curve between luminous intensity and concentration of vitamin B2

